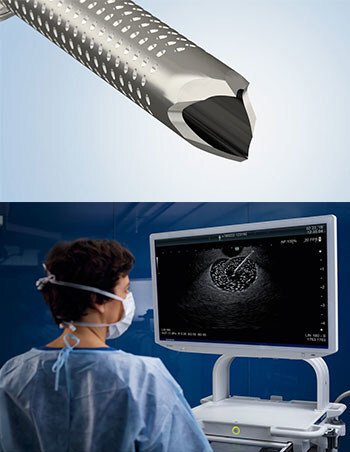
Olympus Corp. of the Americas, a global MedTech company committed to making people's lives healthier, safer and more fulfilling, announced today the U.S. launch of its most advanced single-use fine needle biopsy device used in ultrasound-guided tissue sampling for the diagnosis of diseases such as pancreatic cancer.
The SecureFlex FNB needle is designed to enable the collection of large, intact tissue samples and provide easy access to difficult-to-reach lesions in areas such as the pancreatic head and the uncinate process. Olympus offers the SecureFlex needle in 19G, 22G and 25G sizes.
Olympus will highlight the SecureFlex FNB needle during the Cedars-Sinai Endoscopy Symposium, Jan. 28-31 in Los Angeles and Orlando Live Endoscopy 2026, Feb. 4-6 in Orlando.
EUS-FNB is a technique that combines ultrasound and endoscopy technologies, enabling physicians to obtain tissue or cell biopsies of lesions that cannot be accessed directly by the endoscope. A needle biopsy, for example, may be performed through the wall of the gastrointestinal tract with the ultrasound scope being inserted orally and ultrasound imaging used for sub-mucosal visualisation.
"Gastroenterologists require precision and reliability when performing a biopsy for diagnostic accuracy. Olympus is excited to offer U.S. customers a dependable biopsy solution designed for precision sampling," said Christian Hagie, Vice President, GI EndoTherapy Business Unit Leader, Olympus Corporation of the Americas. "The SecureFlex EUS-FNB needle offers an advanced design to help physicians perform efficient procedures by facilitating access to complex anatomical structures and obtaining adequate samples for diagnostic testing, even in challenging cases."