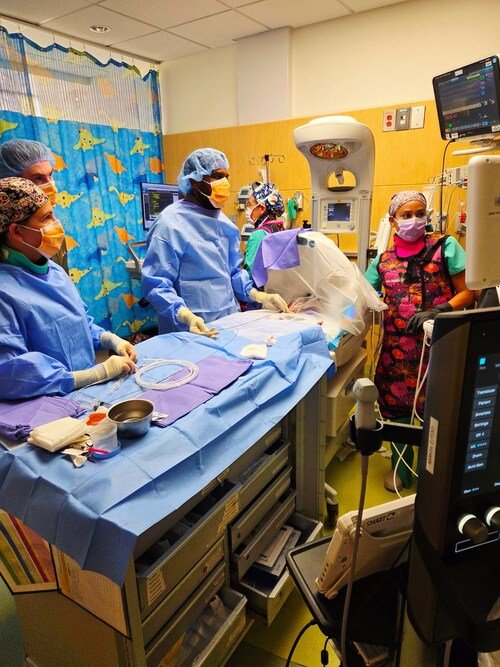
Medtronic has officially enrolled the first patient in its pivotal PEripheral Onyx™ Liquid Embolic (PELE) clinical trial, marking a significant milestone in the evaluation of the Onyx™ Liquid Embolic System (LES) for treating arterial hemorrhage in the peripheral vasculature—areas outside the brain and heart. The inaugural procedure, performed by Dr. Christopher Stark at Albany Medical Center, successfully utilized Onyx LES to control bleeding from a ruptured vessel following a routine medical procedure. This enrollment initiates a larger effort to clinically validate Onyx LES as a viable embolization solution for peripheral arterial hemorrhage in the United States.
The PELE study is a prospective, multi-center, single-arm clinical trial that will enroll up to 119 patients across 25 U.S. sites. Its primary goal is to evaluate the safety and effectiveness of Onyx LES within 30 days of treatment in patients experiencing active arterial bleeding and deemed appropriate for embolization. Onyx LES, already approved for peripheral indications in many markets outside the U.S., offers several procedural advantages, including precise delivery, deep penetration, and long polymerization time, allowing clinicians enhanced control during embolization. This system has demonstrated strong performance in international settings, and the PELE study will help build the clinical foundation necessary to expand its use in the U.S.
This trial not only supports Medtronic’s long-term embolic portfolio strategy but also has the potential to fill a critical unmet need in the U.S. for effective, minimally invasive hemorrhage management tools. If successful, the PELE study could lead to FDA approval of Onyx LES for peripheral indications—broadening treatment options for patients with life-threatening arterial bleeding and empowering interventional radiologists with a proven, adaptable embolic solution. The outcome of this study may ultimately drive wider adoption of liquid embolics in emergency and trauma care, shaping the future of peripheral vascular intervention.