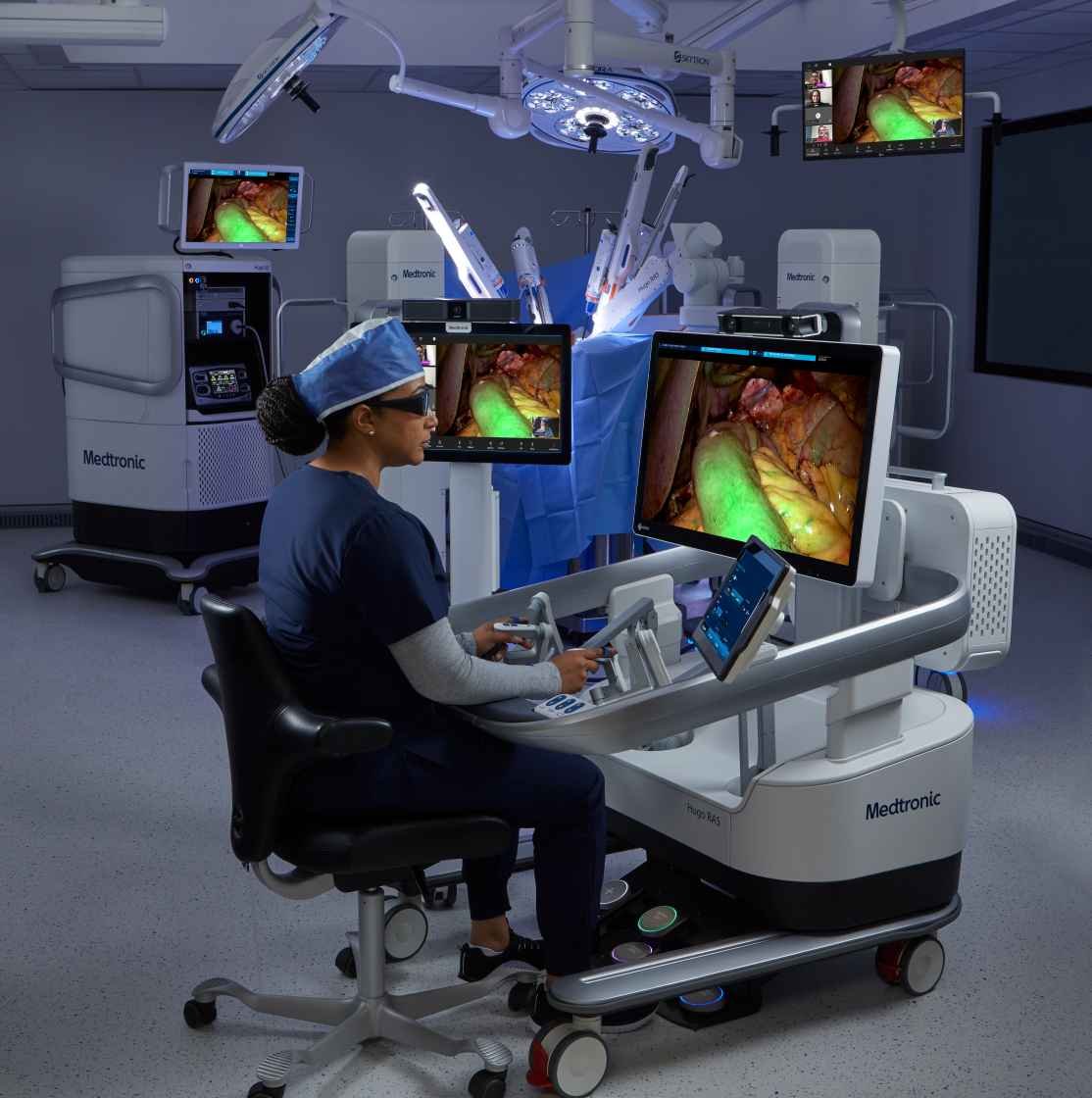
Medtronic has reached a major regulatory milestone with the CE Mark approval of its LigaSure™ RAS vessel-sealing technology for use with the Hugo™ robotic-assisted surgery (RAS) system. This marks the world’s first robotic-driven LigaSure™ instrument, enabling the trusted vessel-sealing capabilities—previously used in over 35 million procedures globally—to be fully integrated into the robotic surgery suite. This development significantly expands the utility of the Hugo™ RAS system for gynecologic, general, and urologic procedures across Europe, reinforcing Medtronic’s mission to bring trusted, advanced technologies into the next generation of minimally invasive surgery.
The LigaSure™ RAS Maryland instrument is specifically engineered for robotic workflows and delivers consistent vessel sealing and tissue cutting with minimal thermal spread. It seals vessels up to 7 mm in diameter within approximately two seconds, thanks to its compatibility with the Valleylab™ FT10 energy platform. The tool enhances surgical efficiency and safety by minimizing collateral damage and offering precise energy delivery during critical moments of a procedure. Surgeons using the Hugo™ system, like Dr. Miguel Caceres, are already noting improved procedural confidence and performance, citing the security of the seal as a significant advancement in robotic energy tools.
This innovation marks a pivotal expansion in Medtronic’s Hugo™ RAS capabilities and underscores the company’s broader strategy to shape the future of surgery through precision robotics, real-time control, and surgeon-centered tools. With plans to introduce telesurgery capabilities and ongoing regulatory review in the U.S., Medtronic is positioning Hugo™ RAS to be a truly global and scalable platform. The integration of LigaSure™ RAS not only elevates clinical versatility but also accelerates Medtronic’s goal to democratize access to advanced robotic solutions—delivering improved outcomes and greater procedural confidence to surgical teams worldwide.