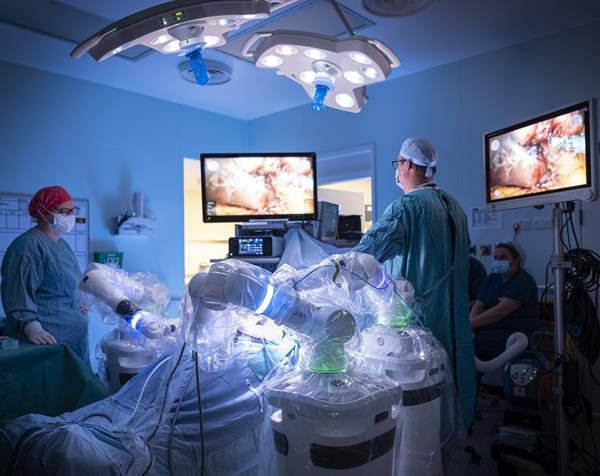
CMR Surgical, a global medical technology company, announces its second-generation surgical robotic platform, Versius Plus, has received 510(k) clearance from the United States Food and Drug Administration (FDA) for cholecystectomy procedures.
With a flexible, modular design and data-driven digital ecosystem, Versius Plus represents a new market offering, providing more choice for surgeons and hospitals. The system brings next-generation innovation to the operating room, designed not only for the surgeon but for the entire OR team. Its open console facilitates communication between the surgeon and other medical professionals, which supports real-time decision making.
The FDA previously granted marketing authorisation for CMR’s first-generation Versius Surgical System through the De Novo process in October 2024. With the 510(k) clearance of Versius Plus, CMR is on track to begin commercialisation in 2026.
Outside of the United States, CMR’s robotic surgical platforms have already completed over 40,000 surgical procedures, establishing a strong foundation for their performance across multiple specialities and care settings, and making the soft tissue robotic platforms the second most utilised globally.
Massimiliano Colella, Chief Executive Officer at CMR Surgical, commented:
“This 510(k) clearance represents an exciting new chapter for CMR Surgical as we introduce Versius Plus to the U.S. market. Built on years of global clinical use data, Versius Plus delivers the flexibility and intelligence today’s healthcare institutions need to advance robotic-assisted surgery. It’s inspiring to see our new technology transforming the landscape of surgical care.”
Chris O’Hara, President & General Manager US at CMR Surgical, commented:
“Versius Plus is designed to meet the practical realities of today’s healthcare environment — adaptable to different settings, efficient to integrate, and scalable for long-term growth. FDA clearance represents an exciting opportunity to partner with healthcare systems across the U.S. Versius Plus is designed to support a broad range of soft-tissue procedures, and we are diligently advancing additional indications in the U.S. and aim to help make robotic-assisted surgery more accessible than ever before.”