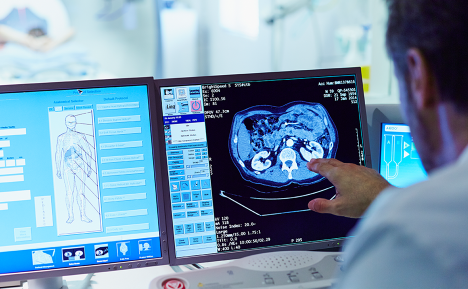
Median Technologies’ eyonis® LCS represents a major leap in early cancer detection, leveraging advanced artificial intelligence and machine learning to improve the accuracy and efficiency of lung cancer screening. As a Software as a Medical Device (SaMD), it is specifically designed for computer-aided detection and diagnosis (CADe/CADx) of lung nodules using low-dose CT scans. Unlike traditional screening tools that rely solely on radiologist interpretation, eyonis® LCS supports clinical decision-making by identifying and characterizing malignant and benign nodules with enhanced precision. This AI-driven approach streamlines the diagnostic workflow, improves standardization, and reduces the risk of missed or misclassified cases, particularly in large-scale population screening programs.
Backed by successful pivotal trials—REALITY and RELIVE—eyonis® LCS has demonstrated both safety and efficacy, reinforcing its value as a reliable screening solution. Its regulatory pathway, including the recent CE Mark Class IIb application in Europe and a 510(k) submission to the U.S. FDA, positions the platform for a near-term commercial launch. With increasing global adoption of lung cancer screening—evident from policy shifts in Germany and pilot programs across the EU—the tool arrives at a critical time. In the U.S. alone, over 14.5 million people are eligible for LDCT-based screening, highlighting the enormous clinical impact eyonis® LCS can have by enabling earlier diagnosis, improving outcomes, and reducing the burden of late-stage lung cancer.
As lung cancer remains one of the leading causes of cancer-related deaths globally, the introduction of eyonis® LCS could mark a paradigm shift in how healthcare systems approach early detection. Median Technologies’ dual regulatory strategy ensures broad accessibility across the U.S. and Europe, offering a scalable and evidence-based solution to support national screening programs. By combining AI innovation with clinical rigor, eyonis® LCS not only enhances diagnostic accuracy but also represents a key advancement in the global fight against lung cancer.