Canada-based Starpax Biopharma Inc. has unveiled its Starpax Cancer Treatment Platform. With a 100% remission rate and no meaningful side effects in all the subjects of the company's preclinical trials, this technology is conceived to address all solid tumours.
The Starpax Magnetodrones are the first component of the Starpax Cancer Treatment Platform. They are living, self-propelled, non-pathogenic bacteria that carry anticancer drugs on their surface and are sensitive to magnetic fields. They are injected directly into a tumor and can swim in tumors without using blood vessels or circulating in the bloodstream.
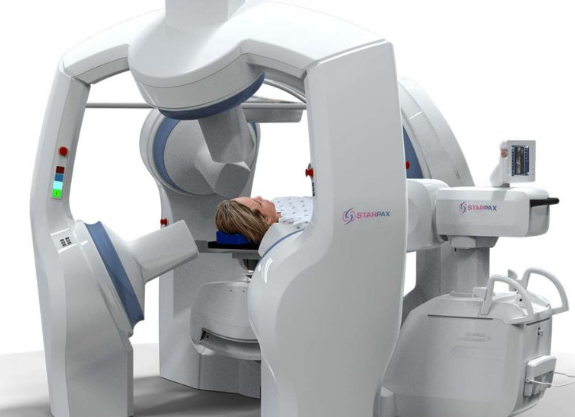
The Starpax PolarTrak is the second component of the platform. It is a proprietary medical device in which the patient is installed. It generates unique magnetic fields to keep the Magnetodrones captive inside the tumor, preventing them from escaping into the rest of the patient's body, and forces the Magnetodrones to spread throughout the whole volume of the tumor, including in hypoxic zones where the cancerous stem cells are located.
The process is managed by the Polartrak artificial intelligence, based on the data of the patient's tumor taken from the MRI imagery scanner days before the treatment. Magnetodrones have faced over 800 mutations in the laboratory and therefore are able to detect low levels of oxygen in the tumor's hypoxia areas where the stem cells are located. Then, they penetrate and accumulate in these areas to introduce the anticancer therapeutic.
To manufacture the Starpax Magnetrodrones, the company has also announced its new facility in Montreal is now in operation. When fully operational, the 27,000-square-foot facility has the capacity to produce Magnetodrones to treat 10,000 patients annually. A second Magnetodrones manufacturing plant is being discussed, with a capacity of 110,000 patients annually.