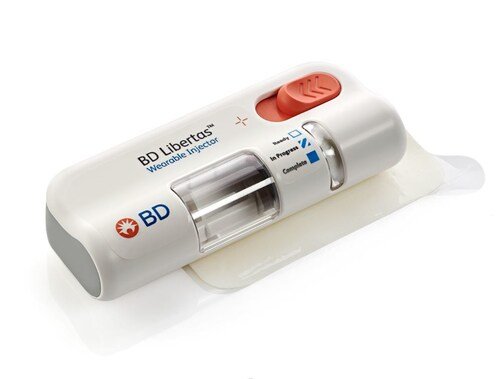
BD’s announcement of the first pharma-sponsored clinical trial using its BD Libertas™ Wearable Injector marks a significant advancement in patient-centered biologic therapy. As more complex biologic drugs enter the market—often requiring hospital-based infusion—the demand for self-administered, subcutaneous delivery systems is growing rapidly. The BD Libertas™ system answers this need with a prefilled, ready-to-use design that eliminates cumbersome setup steps and empowers patients to manage treatment outside of clinical settings. This trial represents a major step forward in validating wearable injectors as a safe, effective alternative to traditional administration methods, reducing the burden on healthcare infrastructure while enhancing patient autonomy.
Designed to support delivery of high-viscosity drugs and large-volume doses (up to 10 mL), the BD Libertas™ Wearable Injector offers flexibility for a broad range of therapies—from oncology and immunology to chronic inflammatory conditions. Its mechanical, user-friendly design simplifies drug administration with a “peel, stick, and click” mechanism, addressing a common barrier to at-home treatment adoption. In BD-led clinical studies, the device demonstrated excellent usability and patient acceptance, with all participants expressing confidence in using the injector if prescribed. For pharmaceutical developers, this system offers a customizable platform that can accelerate time-to-market for combination products and expand access to biologics.
As the global biologics market approaches $670 billion by 2030, drug manufacturers are increasingly seeking integrated delivery solutions that align with evolving patient care models. With robust testing infrastructure, strategic acquisitions like ZebraSci, and strong CMO partnerships, BD is well-positioned to support pharma partners through all stages of development and commercialization. This trial not only reinforces BD’s leadership in advanced drug delivery but also signals a broader shift in healthcare toward self-managed, data-supported treatment paradigms. Looking ahead, the BD Libertas™ platform is poised to play a central role in transforming how complex therapies are delivered, making care more efficient, convenient, and scalable.
MedTech Spectrum's Summary