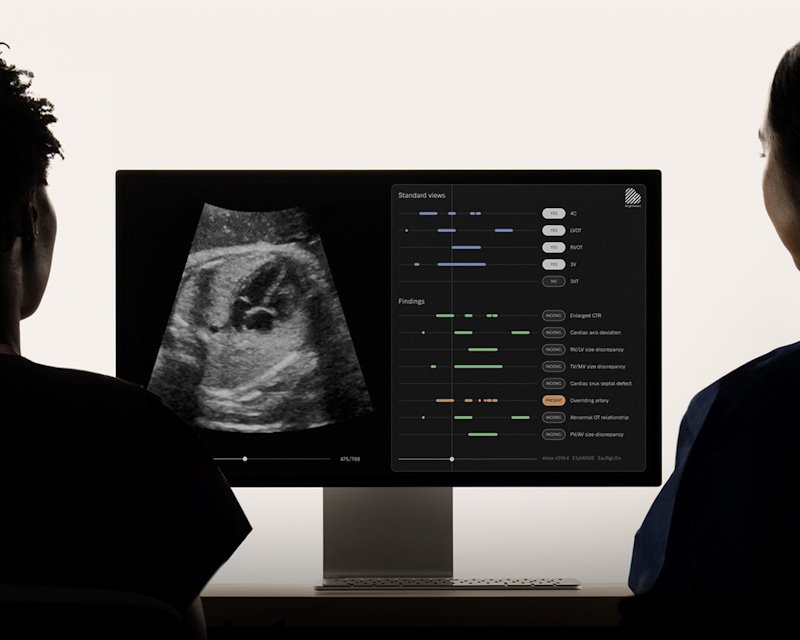
BrightHeart has achieved a significant milestone in obstetric and pediatric cardiology by receiving its third FDA 510(k) clearance, this time for its second AI-powered device, B-Right Views. This innovation marks the company as the first to provide an integrated solution that combines real-time fetal heart documentation with congenital heart defect (CHD) detection. The new software automatically identifies and confirms all standard views required during second and third-trimester anatomy scans, ensuring comprehensive fetal heart assessments regardless of the operator's experience. The integration of this feature into routine ultrasound workflows represents a major leap in the application of AI to one of the most complex aspects of prenatal care.
The importance of this innovation lies in its potential to address long-standing challenges in fetal heart imaging. Historically, variability in sonographer experience and incomplete image capture have led to missed or delayed CHD diagnoses. By offering real-time feedback and view confirmation, B-Right Views can help reduce repeat scans, improve diagnostic accuracy, and provide earlier identification of high-risk cases. The FDA's approval of BrightHeart’s Predetermined Change Control Plan (PCCP) further sets the company apart, allowing it to rapidly implement AI improvements without requiring separate regulatory submissions—accelerating innovation and clinical impact.
BrightHeart’s full AI platform now includes B-Right Screen and B-Right Views, delivering a unified system that enhances sonographer confidence, streamlines workflows, and promotes earlier triage of fetal cardiac anomalies. The company is preparing for a limited market release to build on its successful pilot program and invites collaboration with clinicians, researchers, and industry partners. With its technology poised to reshape prenatal care, BrightHeart continues to lead the transformation toward smarter, more consistent, and accessible maternal-fetal health solutions.
MedTech Spetrum's Summary
Innovation: BrightHeart received its third FDA 510(k) clearance for B-Right Views, an AI-powered tool that automatically detects and confirms all required fetal heart views during second and third-trimester ultrasounds, ensuring complete and accurate documentation.
Importance & Usage: The integrated solution enhances diagnostic accuracy and workflow by providing real-time feedback during exams, reducing the need for repeat scans and helping sonographers identify potential congenital heart defects (CHDs) regardless of experience level.
Conclusion: With its third clearance and approval of a Predetermined Change Control Plan (PCCP), BrightHeart leads in AI-enabled prenatal care, preparing for broader clinical adoption and setting a new standard for maternal-fetal health innovation.