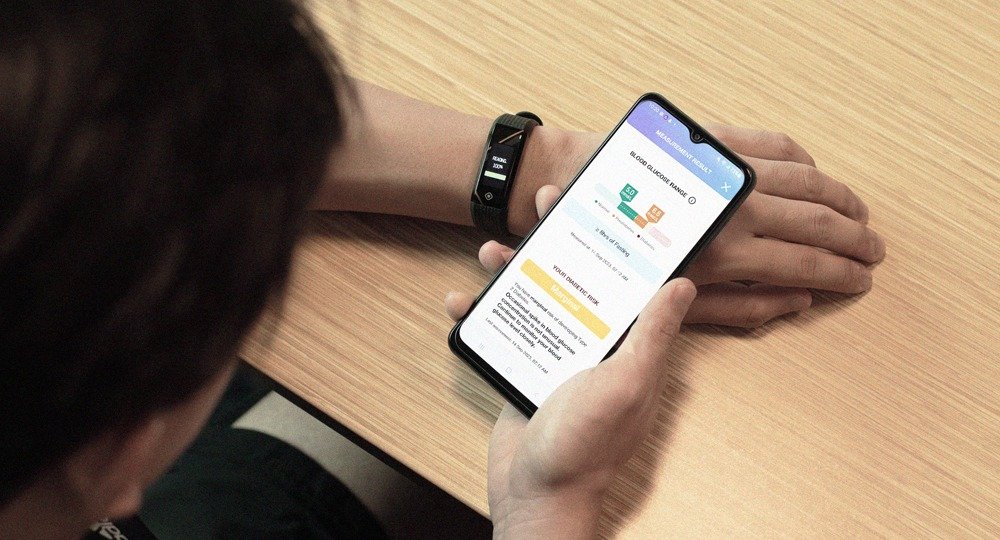
Actxa Pte Ltd, a Singapore-based deep tech company, introduces its Blood Glucose Evaluation & Monitoring (BGEM) technology. Powered by a cloud-based AI-driven algorithm, BGEM offers a non-invasive solution for blood glucose monitoring, marking a new approach to early diabetes detection and management. Diabetes: A Growing Concern In Singapore, it is projected that by 2050, at least 1 million people will be living with diabetes, with 1 out of 4 remaining undiagnosed. Globally, the number of diabetic individuals is expected to reach about 643 million by 2030, with 1 in 2 adults undiagnosed. Undiagnosed diabetes poses severe risks, as early-stage symptoms are often silent, leading to delayed diagnosis and potentially irreversible complications. Regular glucose monitoring is essential for detecting the risk of diabetes early, which enables timely and life-saving interventions.
A Non-Invasive Approach to Blood Glucose Monitoring BGEM leverages advanced photoplethysmography (PPG) sensors to offer a non-invasive blood glucose monitoring solution. By integrating this technology into wearables, BGEM allows users to monitor their diabetic risk assessment regularly. This could support and promote early intervention, leading to better health outcomes while reducing long-term pain and cost for individuals. A joint study by Actxa and KK Women’s and Children’s Hospital (“KKH”), titled “Utility of photoplethysmography in detecting elevated blood glucose among non-diabetics” and published in the Singapore Medical Journal, validates the effectiveness of PPG sensors in its non-invasive blood glucose detection. The study highlights BGEM’s capabilities in integrating wearable devices with PPG sensors to assess the risk of impaired glucose regulation. The full study can be accessed.
Clinical Validation and Global Partnerships BGEM underwent its first clinical trials in 2022, with its first scientific paper published in 2023. The technology has garnered attention for its potential in non-invasive blood glucose monitoring. Actxa continues to collaborate with international partners to explore the broader potential of BGEM, bringing it to markets globally while remaining committed to advancing local innovations in Singapore. Alleviating Financial Strain Through Diabetes Prevention With diabetes on the rise globally, the focus is shifting from reactive treatment to proactive prevention. BGEM’s early detection capabilities enable individuals to make lifestyle changes that can prevent or delay the onset of Type 2 diabetes mellitus (T2DM).
By reducing the burden of long-term diabetes management, BGEM contributes to healthier populations and more sustainable healthcare systems. In Singapore, the healthcare system faces significant strain due to the rising number of diabetes cases, with around $940 million spent on diabetes-related care in 2017. By enabling earlier detection and intervention, BGEM has the potential to ease this burden, contributing to more sustainable healthcare outcomes. Innovating for a Healthier Future Marcus Soo, Chief Executive Officer of Actxa, said, “As a company with a vision to help people live healthier and longer lives through technology, we are dedicated to translating technological innovations into evident solutions that enhance lives. BGEM simplifies the monitoring process, allowing users to gain insights into their health regularly and noninvasively. By continuing to collaborate with partners and refine our technology, we hope to empower more individuals to manage their health proactively.
We believe this can lead to better outcomes and support the sustainability of healthcare systems.” Actxa is committed to improving the lives of those affected by diabetes hrough cutting-edge technology and meaningful collaborations.