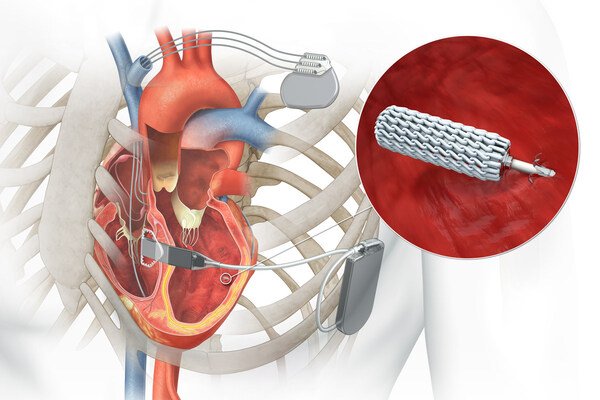
EBR Systems, Inc. has received FDA approval of the WiSE® System, which marks a significant leap forward in the treatment of heart failure. Unlike conventional CRT, the WiSE System is the first and only to deliver leadless left ventricular endocardial pacing (LVEP), that closely aligns with the heart's natural conduction pathway. The endocardial approach represents a more physiological method of resynchronization and allows electrophysiologists to treat patients that are not able to receive lead-based devices. This advancement offers a pioneering treatment option for patients who have limited access to conventional CRT therapies.
Expanding Access to Patients
Conventional CRT leaves too many heart failure patients behind. The WiSE System was designed for them. It allows physicians to help these key patient populations:
In short: WiSE brings CRT to more patients than ever before.
Backed by Data. Reimagining Delivery of CRT.
The SOLVE-CRT trial has delivered promising results for heart failure patients. The WiSE System offers hope to those who have tried other therapies without success and were told no further options existed—until now.
Seamless Integration.
The WiSE System syncs with existing pacing devices—pacemakers, ICDs, or CRTs—using a subcutaneous ultrasound Transmitter to power an ultra-compact Electrode implanted in the LV.
No leads. No lead navigation. Just endocardial pacing that's more physiologic, from the inside out.
Empowering Physicians, Powering Hearts.
"We're delighted for the heart failure patients who were not treatable with existing CRT lead-based devices," said John McCutcheon, President and CEO of EBR Systems. "This milestone celebrates the dedication of our team, the support of our shareholders, and empowers electrophysiologists with a vital new tool to help their patients. It marks the culmination of EBR's 22-year pre-commercial phase and the start of our journey as a high-growth, medical device company."