Geneoscopy’s latest FDA approval marks a meaningful innovation in at-home colorectal cancer (CRC) screening, simplifying one of the most uncomfortable aspects of stool-based testing. The updated stool collection kit for ColoSense®, Geneoscopy’s RNA-based CRC screening test, eliminates the need for sample scraping and multiple containers, making the process more user-friendly. By streamlining collection without compromising clinical performance, this improvement is poised to overcome a key barrier to patient adherence - particularly for those hesitant about traditional stool-based methods. In doing so, the company addresses a critical access challenge in CRC screening among the more than 44 million average-risk individuals aged 45 to 75 who remain unscreened.
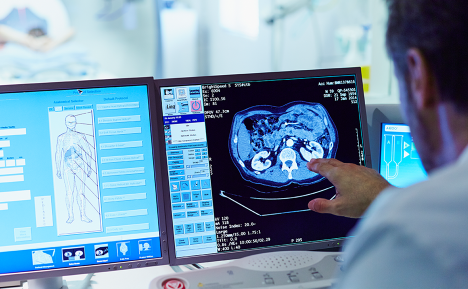
ColoSense stands out as the only FDA-approved RNA-based test for CRC, offering high sensitivity for both cancer and advanced adenomas. The test’s ability to detect early-stage colorectal cancer, including in individuals aged 45-49-a population seeing rising incidence rates-is particularly impactful. Its inclusion in NCCN guidelines and its distribution partnership with Labcorp position it as a powerful tool in national CRC prevention efforts. The improved usability of the test further strengthens its potential as a first-line, non-invasive option that aligns with patient preferences for at-home, high-sensitivity diagnostics.
Looking ahead, Geneoscopy’s enhanced ColoSense test reflects a broader movement toward more accessible, patient-centered diagnostics in cancer screening. By focusing on the user experience and integrating proven RNA biomarker science, the company is not only expanding participation in early detection but also setting a new standard for how preventive care can be delivered at scale. As health systems and public health programs aim to raise screening adherence rates, tools like ColoSense—built for both clinical rigor and patient comfort-will be instrumental in driving down CRC mortality through earlier, more widespread detection.