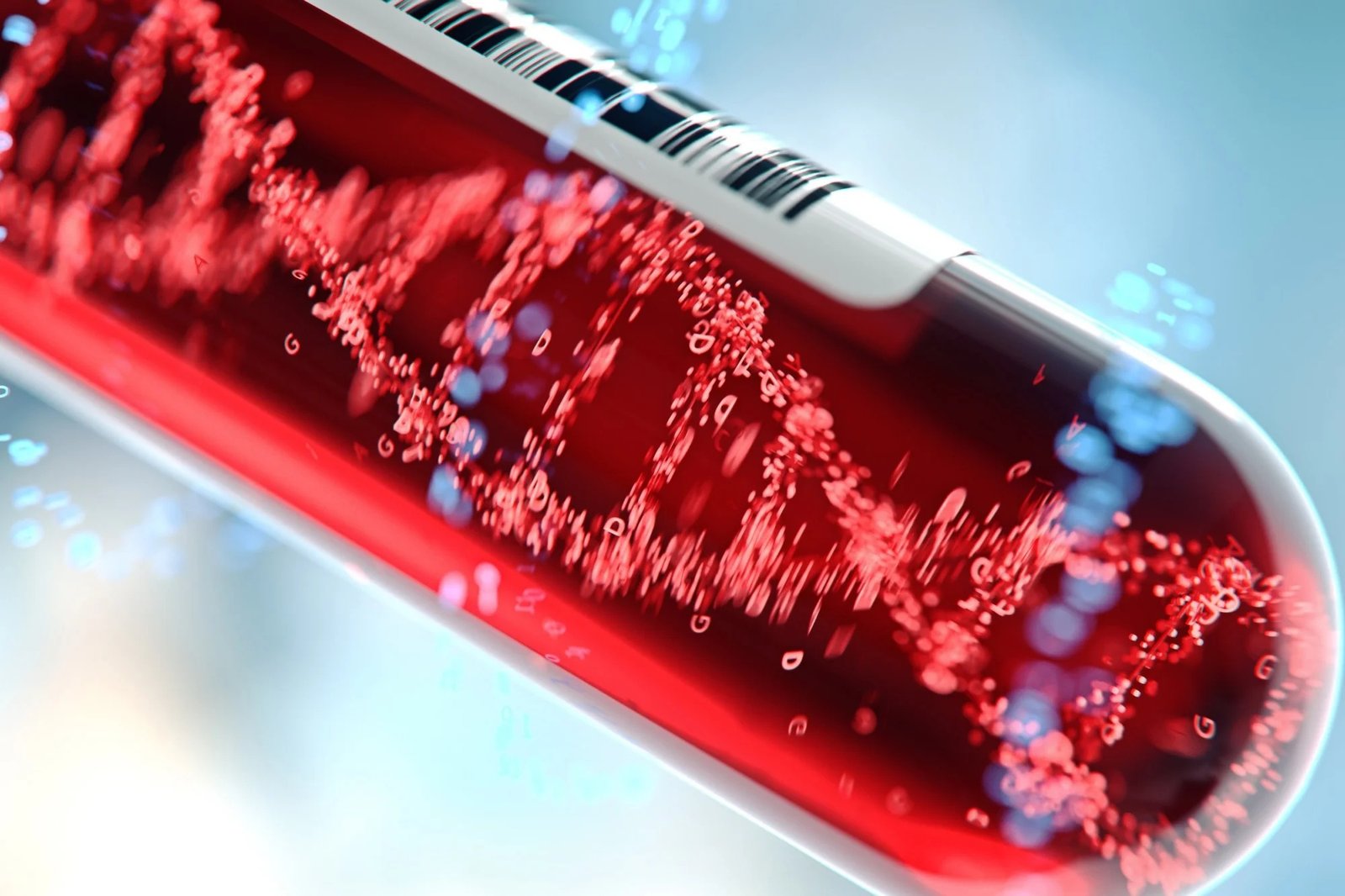
Human Longevity, Inc. (HLI) has expanded its cancer prevention program by incorporating the ClearNote Health Avantect® Pancreatic Cancer Test into its offerings for executive health clients. This advanced, DNA-based blood test detects early-stage pancreatic cancer by analyzing epigenomic markers in cell-free DNA. When combined with HLI’s proprietary whole genome sequencing and high-resolution MRI imaging, the test forms part of one of the most comprehensive early cancer detection strategies available.
The Avantect test is intended for high-risk individuals, such as those over 50 with new-onset diabetes, genetic predispositions like BRCA2 mutations, or a family history of pancreatic cancer. It demonstrated 68.3% sensitivity and 96.9% specificity for early-stage detection, offering a non-invasive yet highly accurate tool to catch cancer when intervention is most effective. This integration aligns with HLI’s goal of identifying diseases before symptoms emerge, improving patient outcomes through proactive health management.
This move also reflects Human Longevity’s broader mission to extend healthspan through early, personalized detection and intervention. Executive clients already participating in programs targeting key health risks will now have access to the Avantect test, further enhancing HLI's reputation as a leader in precision medicine. As Dr. Wei-Wu He, Executive Chairman of HLI, noted, combining genomics, epigenomics, and imaging enables early detection that can truly change lives.
MedTech Spectrum's Summary