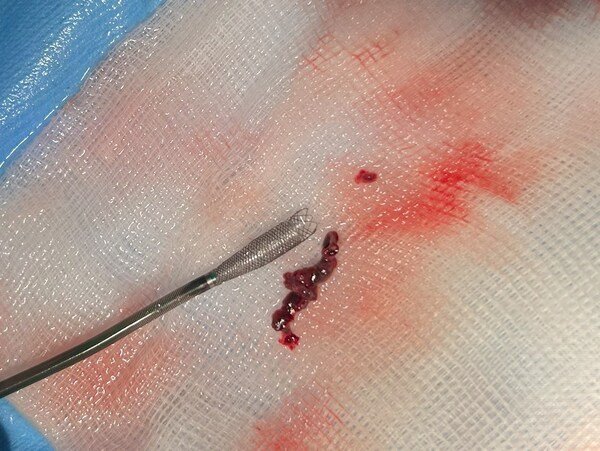
image caption: A brain clot removed with Ceretrieve’s device
Ceretrieve announced the successful results of the company's multicenter, single-arm study, showcasing the capabilities of its state-of-the-art aspiration catheter. The study, conducted across two centers, included 20 patients who suffered from acute ischemic stroke (AIS) due to intracranial large vessel occlusion (LVO) and were eligible for thrombectomy within 24 hours of symptom onset. The study was aimed for assessing the safety and initial performance of the Ceretrieve Device.
Designed for seamless integration within a conventional 6Fr delivery catheter, Ceretrieve's device offers trackability and maneuverability, ensuring efficient access to the clot location. Ceretrieve's catheter’s aspiration capabilities, in comparison to existing devices, allows for clot(s) removal in a single pass, while fully restoring blood flow. In the study conducted, Ceretrieve's aspiration catheter ensured the highest safety standards by reducing the risk of releasing clot fragments further into the brain. In the first-in-human (FIH) study 80% Complete/Near-Complete Perfusion was achieved.
Prof. Serder Geyik, MD (Florya Medical Park, Turkey) said, "Ceretrieve's device aspirates the clot with a giant bore tip- double than the largest catheters in the market, for maximal vacuum effect without paying the price in trackability. In addition, it provides local flow restriction in the middle cerebral artery reducing distal emboli. It is the only device that includes all the features for successful thrombectomy and first pass effect in one single device."