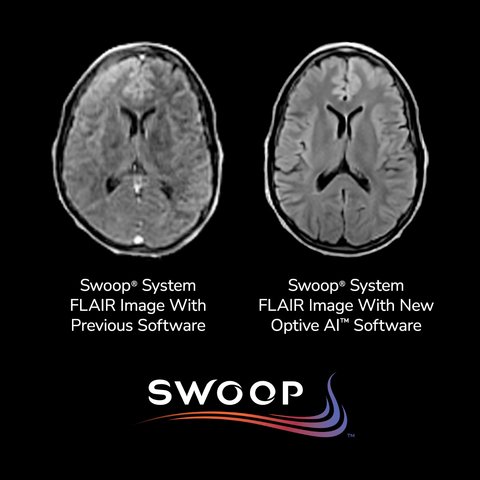
Hyperfine’s recent FDA clearance of its tenth-generation software, Optive AI™, marks a transformative leap forward in portable brain imaging technology. Integrated with the Swoop® Portable MR Imaging® system—the world’s first FDA-cleared AI-powered portable MRI—Optive AI™ uses advanced artificial intelligence algorithms to significantly enhance image quality in ultra-low-field magnetic resonance imaging. This innovation addresses a longstanding limitation in portable MRI by delivering clearer, more uniform, and anatomically detailed images, a breakthrough that brings ultra-low-field performance closer to that of conventional 1.5T scanners.
In clinical usage, Optive AI™ enhances every phase of the imaging process, from noise reduction and image acquisition to reconstruction and post-processing. This results in faster, more confident diagnostic decisions at the point of care, especially in emergency, bedside, and resource-limited settings. Early feedback from clinical sites has been overwhelmingly positive, with radiologists noting a marked improvement in image clarity and diagnostic confidence. These enhancements are critical for applications such as stroke detection, trauma assessment, and neurological monitoring, particularly when access to full-scale MRI units is delayed or impractical.
The importance of this advancement lies in its potential to redefine where and how brain imaging can occur. By improving diagnostic accuracy and expanding the range of environments in which MRI can be performed, Optive AI™ helps democratize access to high-quality imaging. The software’s rollout, expected in Q3 2025, supports Hyperfine’s broader growth strategy into hospital networks and neurology practices, ensuring more patients benefit from timely and accurate diagnoses. In conclusion, Optive AI™ represents not just a software upgrade, but a major milestone in the evolution of accessible, AI-powered neuroimaging.