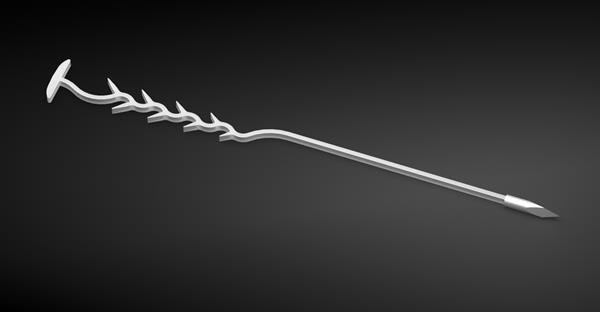
Spirair, Inc. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its TurbAlign™ Implant, a bioabsorbable device designed to aid recovery following sinus surgery. As a minimally invasive solution, TurbAlign addresses a key challenge in otolaryngology—maintaining proper anatomical alignment of nasal structures during healing. The implant works by gently separating the middle turbinate from the lateral nasal wall, reducing the risk of adhesions and tissue displacement that can compromise surgical outcomes. With its innovative design and resorbable material, TurbAlign eliminates the need for implant removal, enhancing procedural efficiency and patient comfort.
TurbAlign is deployed using a simple, knot-free, one-pass technique, offering ENT specialists a streamlined method for securing the middle turbinate during the critical post-operative period. The implant supports stable medialization of the turbinate, promoting optimal airflow and minimizing complications such as scarring or blockage that often occur after routine sinus procedures. Clinicians, including Stanford Medicine's Dr. Jayakar Nayak, have highlighted the importance of maintaining nasal structure alignment to ensure long-term surgical success, and TurbAlign offers a practical, evidence-informed tool to meet this clinical need.
With commercial availability expected in the U.S. later this year, TurbAlign marks a strategic expansion of Spirair’s ENT-focused device portfolio. Paired with its previously introduced SeptAlign™ device, which addresses septal deviation, the company now offers complementary technologies aimed at improving outcomes in nasal and sinus surgery. As demand grows for solutions that enhance post-surgical healing while reducing procedural complexity, Spirair’s innovations are well-positioned to deliver meaningful benefits to both clinicians and patients, ultimately advancing the standard of care in otolaryngology.
MedTech Spectrum's Summary
FDA Clearance Validates Innovation: Spirair’s TurbAlign™ has received 510(k) clearance from the FDA, affirming its safety and effectiveness as a novel, bioabsorbable implant designed to improve healing outcomes after sinus surgery.
Improved Surgical Recovery: The implant minimizes post-operative complications such as tissue adhesion and turbinate displacement by maintaining proper nasal structure alignment—enhancing airflow, healing, and overall surgical success.
Advancing ENT Care: With TurbAlign and its complementary SeptAlign™ device, Spirair strengthens its ENT portfolio, offering minimally invasive, easy-to-use solutions that support better patient outcomes and long-term care in sinus and nasal procedures.