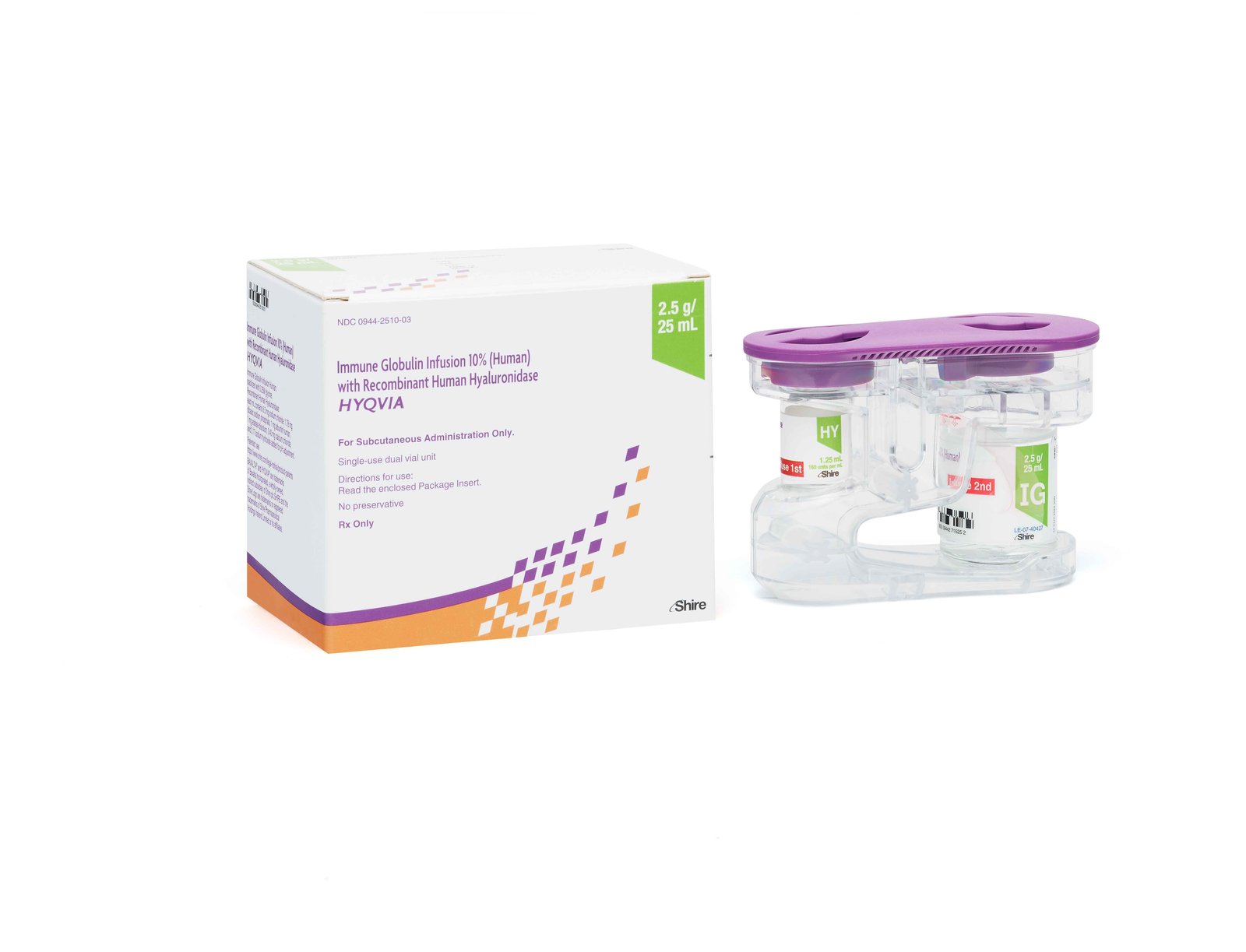
Takeda has received FDA 510(k) clearance for its HyHub™ and HyHub™ Duo devices, marking a significant advancement in patient-centered solutions for subcutaneous immunoglobulin (SCIg) therapy. Designed specifically for use with HYQVIA®, these novel devices allow patients 17 years and older to transfer dual vial units (DVUs) of immunoglobulin (IG) and hyaluronidase without needles—streamlining the infusion process in both home and clinical environments. This regulatory milestone supports Takeda’s continued efforts to simplify complex therapies and reflects the company’s strategic focus on innovation in its plasma-derived therapies portfolio.
The HyHub and HyHub Duo systems serve as intuitive docking stations that reduce the number of preparation steps needed for HYQVIA administration by up to 50% when compared to traditional pooling bag methods. These devices also minimize the use of ancillary supplies and offer convenient features, such as dedicated carrier bags for enhanced mobility during treatment. Developed with direct input from patients and caregivers, the devices address a critical need for ease of use, especially for individuals with primary immunodeficiency (PI) and chronic inflammatory demyelinating polyneuropathy (CIDP), who require regular and often lifelong infusions.
Looking ahead, Takeda plans to launch HyHub and HyHub Duo in the United States during the second half of fiscal year 2025 and has submitted a CE Mark application to expand access across the European Union. With the potential to improve adherence, comfort, and autonomy for patients, these devices represent a promising step forward in the evolution of home-based infusion therapy. By offering a simplified, needle-free experience tailored to HYQVIA, Takeda reinforces its commitment to a patient-centric ecosystem that enhances both outcomes and quality of life for those managing immune and neurological disorders.