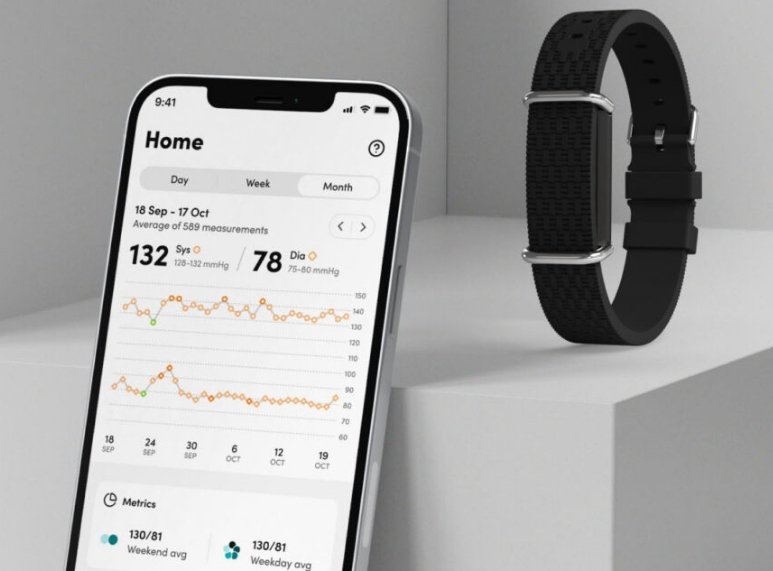
Aktiia, the Swiss startup revolutionising Blood Pressure (BP) monitoring and management, has received regulatory approval (CE mark) for its next-generation optical technology that does not require calibration with a traditional cuff. The CE mark allows market access to potentially over 40 countries.
The underlying calibration-free technology, known as CALFREE, uses input from optical sensors commonly found in either smartwatches or the cameras of commercial smartphones. This can enable integration of Aktiia's CALFREE technology into various third-party devices.
Aktiia's CALFREE technology offers accessible, comfortable, and convenient method for long-term blood pressure monitoring.
Since its inception in 2018, Aktiia has been an innovative pioneer of blood pressure technology, known for its scientific rigor, visionary team, and initial breakthrough product, the first continuous BP monitoring device worn on the wrist, which currently requires monthly calibration. This announcement represents a significant advancement in BP data collection and hypertension management, enabling the development of optical systems that do not require any calibration while meeting the stringent BP performance thresholds for regulatory approval.