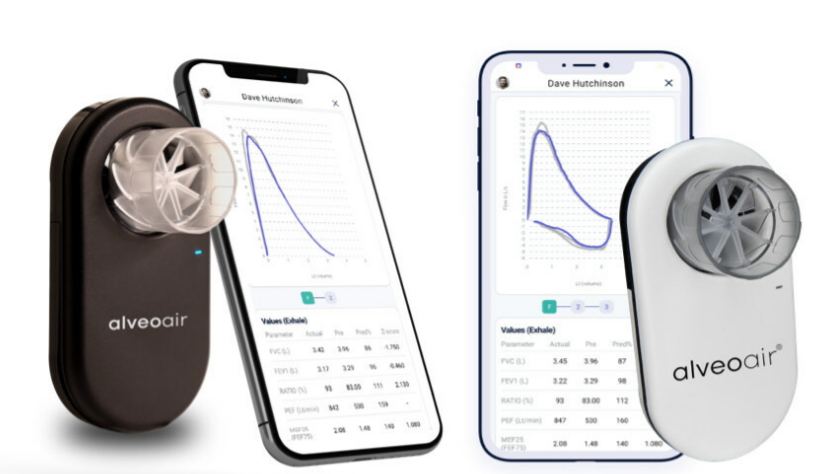
Alveofit (Roundworks Technologies Private Limited), a ( India ) , Pune-based startup, has announced that its groundbreaking product, alveoair spirometer, has earned US FDA clearance for distribution in the United States. Specialising in digital therapeutics for respiratory care, the company is committed to delivering affordable and interoperable lung health solutions. Alveoair offers real-time, valuable insights to healthcare professionals, enabling timely interventions in respiratory care.
This US FDA approval, coupled with strategic partnerships like the one with AstraZeneca, positions alveofit for broader international expansion, particularly in the US and emerging economies.
Since achieving Central Drugs Standard Control Organisation (CDSCO) certification in India in June 2022, alveoair has been adopted by over 400 healthcare facilities across India. Partnerships with organisations like India Sweden Innovation Centre (ISIHC), NASSCOM COE, PATH, Forge Innovation & Ventures, and DST have helped positively impact the lives of thousands of patients.
Furthermore, Alveofit collaborates with prominent academic institutions such as AIIMS Delhi for clinical research and has formed an alliance with Tatvacare to extend digital respiratory care solutions to patients' homes.
Earlier this year, to cater to the growing US market, Alveofit inaugurated its office in New York.