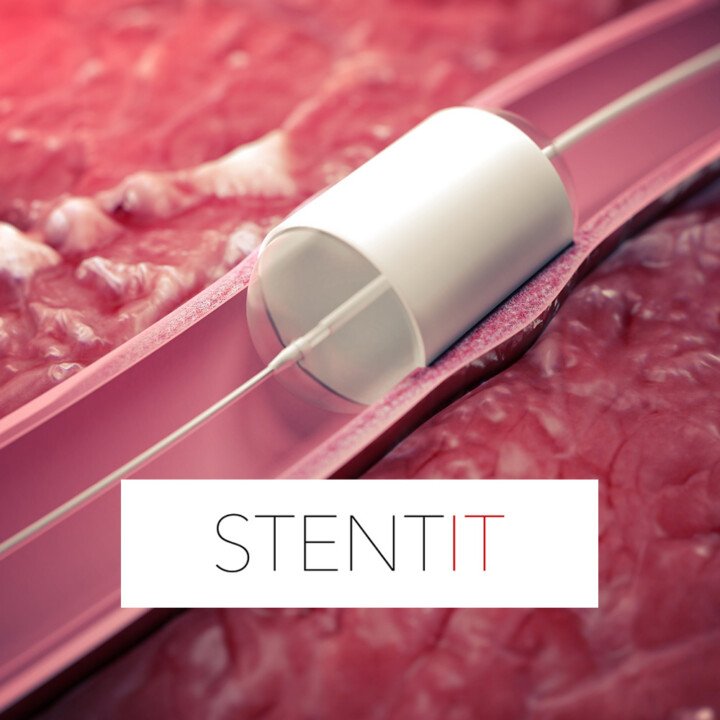
STENTiT’s successful first-in-human implantation of its Resorbable Fibrillated Scaffold (RFS) marks a groundbreaking advancement in vascular medicine, particularly for patients suffering from chronic limb-threatening ischemia (CLTI). Unlike conventional metallic stents that remain permanently in the artery, the RFS is bioresorbable and composed of microfiber scaffolding that provides temporary structural support while actively guiding the regeneration of arterial tissue. This innovation introduces a new paradigm in endovascular intervention—one that not only restores blood flow but also promotes healing from within by leveraging the body’s own regenerative capacity.
The usage of RFS in below-the-knee CLTI cases addresses one of the most challenging areas in peripheral artery disease (PAD) management, where patients are at high risk of limb amputation and recurrent procedures. By allowing cellular infiltration through its porous design, the scaffold encourages neotissue formation, gradually restoring native vascular function. As the synthetic structure dissolves naturally over time, it reduces long-term risks such as inflammation, restenosis, and complications associated with permanent implants, offering a more holistic and patient-centric approach to vascular repair.
This innovation is of particular importance as the burden of PAD continues to rise globally, and current treatment options often fail to provide durable outcomes. The VITAL-IT 1 clinical trial, now underway at the Medical University of Graz, Austria, aims to validate the safety, feasibility, and long-term benefits of the RFS technology in a high-risk patient population. If successful, STENTiT’s regenerative stent could set a new standard in vascular care by offering a dynamic solution that merges mechanical precision with biological healing—ultimately improving quality of life and reducing the need for repeat interventions.