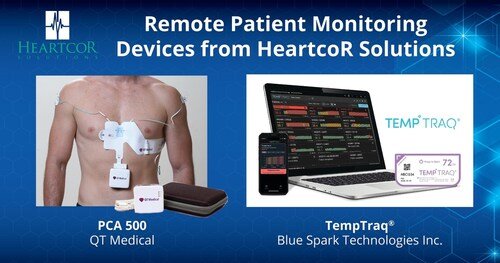
HeartcoR Solutions, a leading core lab focused on the collection, analysis, and reporting of cardiac safety and other physiological data in clinical trials, announced today exciting growth in its suite of remote monitoring technology. The addition of the TempTraq device from Blue Spark Technologies, Inc., and the PCA 500 from QT Medical allows HeartcoR to create a one-stop shop for clinical trial sponsors.
"Healthcare innovation relies on the ability to prove the safety and efficacy of new medical devices and therapeutics," said Ciaran Cooper, CEO of HeartcoR Solutions. "By expanding our suite of remote monitoring devices, we're empowering clinical trial sponsors to save time and streamline workflows by sourcing all of their device needs through one partner, HeartcoR Solutions."
The rising demand for more inclusive, patient-centric healthcare, including increased convenience and access, has led the pharmaceutical and medical device industries to seek out a growing number of remote monitoring solutions when collecting vital data on the safety and efficacy of innovative, new interventions in clinical trials.
The global RPM market is projected to grow at a CAGR of 19.8 per cent from 2025 to 2033. This reflects an unprecedented number of new devices reaching the market over the next several years, ranging from consumer-based at-home monitoring devices to sophisticated patches worn under physician supervision for a range of physiological measurements. To capitalise on this opportunity, clinical trial sponsors must be able to efficiently source devices for different physiological measures. By expanding its suite of RPM devices, HeartcoR Solutions aims to improve access to and convenience for subjects in clinical trials, thus ultimately driving better data while streamlining processes and reducing overall trial costs.