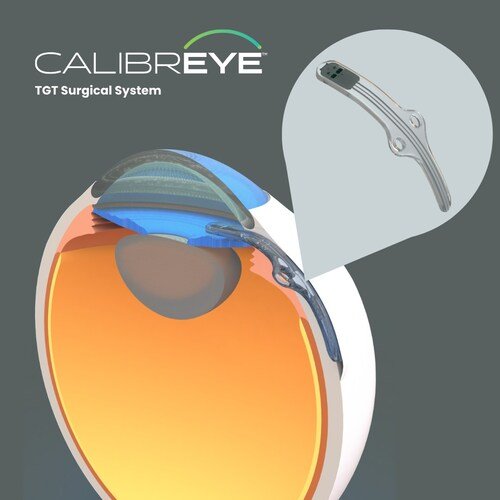
Myra Vision, a Shifamed portfolio company based in California, has received conditional approval from the U.S. Food and Drug Administration (FDA) to initiate its Investigational Device Exemption (IDE) study, known as the ADAPT trial. This prospective, nonrandomized, open-label study will evaluate the safety and effectiveness of the company’s Calibreye™ Titratable Glaucoma Therapy™ (TGT) Surgical System in up to 70 refractory glaucoma patients, with the primary effectiveness endpoint set at 12 months.
Glaucoma, a leading cause of irreversible blindness affecting an estimated 80 million people globally, currently has no cure. The only proven method to slow or prevent optic nerve damage is through lowering intraocular pressure (IOP). However, current therapies are not always effective, and surgical approaches such as trabeculectomy or tube shunts can carry risks without offering the ability to adjust treatment after implantation. The Calibreye system aims to change this by enabling ophthalmologists to personalize therapy with adjustable and reversible aqueous outflow control, potentially improving outcomes while minimizing complications.
The device is designed to be implanted once, after which its outflow settings can be adjusted through a slit-lamp examination as patient needs evolve. According to Robert Chang, President and CEO of Myra Vision, the FDA’s approval represents a major milestone for the company and the broader glaucoma care field. Early clinical experiences with the technology have been encouraging, and the upcoming U.S. trial will be critical in determining its potential to address an urgent unmet need in ophthalmology.
MedTech Summary
Myra Vision receives FDA conditional IDE approval to start the ADAPT trial for its Calibreye™ glaucoma surgical system.
The technology offers adjustable, reversible outflow control to optimize intraocular pressure management.
Trial to enroll up to 70 refractory glaucoma patients, with results expected after a 12-month evaluation period.