Antengene Corporation Limited, a leading innovative, commercial-stage global biopharmaceutical company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class medicines for cancer, announced that XPOVIO® (selinexor) in combination with bortezomib and dexamethasone (XVd) for the treatment of adult patients with relapsed/refractory multiple myeloma (R/R MM) who have received at least two prior therapies, has been approved for reimbursement in Taiwan. Starting from March 1, 2025, XPOVIO® will be officially included in the NHI drug reimbursement scheme.
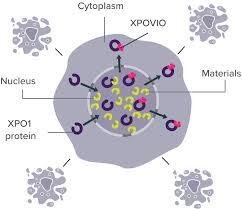
With a novel mechanism of action, XPOVIO® is the world's first approved orally-available, selective XPO1 inhibitor, which has already been approved in nine countries and regions in APAC, and included in the public insurance schemes in five of those markets (the mainland of China, Taiwan market, Australia, Singapore and South Korea). Moving forward, XPOVIO® is expected to extend public health insurance coverage across APAC markets.
Multiple myeloma (MM) is a malignancy caused by the dysregulated proliferation of plasma cells. According to epidemiology data, MM is the second most prevalent hematologic malignancy in Taiwan market, accounting for approximately 700 to 800 newly diagnosed cases and around 400 relevant deaths each year.[1] Most patients with MM have to face a range of challenges in treatment, including high propensity to relapse, short period of survival, and limited treatment options. The inclusion of XPOVIO® for reimbursement coverage in Taiwan market, will further reduce the financial burden on many patients, benefiting more patients and their families.
While bringing XPOVIO® to more APAC markets, Antengene is also striving to expand the indications of XPOVIO®. Leveraging the drug's novel mechanism of action, XPOVIO® is currently being developed with multiple combination regimens for the treatment of various additional indications including myelofibrosis (MF) and endometrial cancer.