The AeviceMD received U.S. FDA 510(k) clearance as a Class II medical device. ● The AeviceMD is part of a broader system that continuously monitors biomarkers of interest to detect early signs of respiratory exacerbation.
● Coronet Ventures, the international Cedars-Sinai organization, along with East Ventures, a leading sector-agnostic venture capital firm in Southeast Asia, will be participating in Aevice Health's bridging round.
This clearance empowers the company to market and provide their novel remote monitoring platform to health systems across the country, expanding access to a hassle-free and convenient solution for assessing lung health in clinical and home settings.
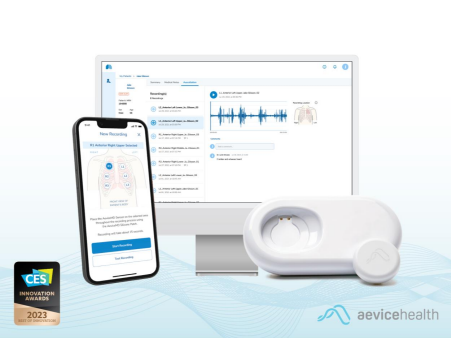
The AeviceMD is an integral part of the broader AeviceMD Monitoring System, a comprehensive patient management platform targeted towards chronic respiratory disease management. Leveraging cutting-edge proprietary algorithms, the AeviceMD Monitoring System continuously monitors biomarkers of interest acquired by its smart wearable stethoscope and detects early signs of respiratory exacerbation. The robust platform has completed trials in the emergency department for the analysis of abnormal lung sounds like wheezing against the current standard of care — the clinicians’ own stethoscope-based analysis. The results from these trials are currently being collated and will be disclosed in a separate and forthcoming announcement.
Asthma and COPD, the two most common chronic respiratory diseases, account for over 41 million patients in the US, with authorities expecting the actual prevalence to be higher due to underdiagnosis. These diseases contribute significantly to hospital emergency department visits and readmissions, accounting for over $130B1,2in direct and indirect costs for the country. Lack of access to post-discharge care and follow-ups are key drivers of the hospitalizations and readmissions.
With the AeviceMD, the clinic can now be brought home. The AeviceMD elevates the ubiquitous stethoscope - an essential tool in every clinic - into a smart, digital, and easily accessible device. Caregivers and patients can simply conduct auscultations using the AeviceMD sensor and conveniently share them with their healthcare professional. Through analysis of these recordings, healthcare providers can accurately identify abnormalities and promptly advise patients, enabling proactive management of lung health.
“This FDA clearance represents a remarkable milestone for our company. While diseases like diabetes or heart failure have seen advancements in technology, there remains a notable lack of comprehensive solutions for respiratory conditions. Unfortunately, the patients requiring these solutions the most are often the ones facing challenges in accessing adequate care. With this clearance, we take a significant stride towards becoming the equivalent of continuous glucose monitors for diabetes, but for respiratory health — a patient centric, affordable, and accessible solution that empowers patients to achieve a healthy recovery from the comfort of their homes,” said Adrian Ang, CEO of Aevice Health.
As a participant in the Cedars-Sinai Accelerator Class 8, Aevice Health has received invaluable support from the program during its developmental journey in the US. Over the course of the three-month program, the company underwent rigorous mentorship, refining its product-market fit for the US market and strategically shaping its product launch strategy. This was made possible with guidance from clinical key opinion leaders, executive management, operations members of the health system, and investors. As part of their plans, the company is working towards a pilot program with the Cedars-Sinai Medical Center.
Additionally, Coronet Ventures, the international Cedars-Sinai organization, and East Ventures, a leading sector-agnostic venture capital firm in Southeast Asia, will be joining Aevice Health’s bridging round with an undisclosed investment.
2023 has been a momentous year for the AeviceMD thus far. This announcement comes at the heels of the company’s recent Singapore Health Sciences Authority (HSA) first in-market approval for the AeviceMD Monitoring System in March. Earlier in January, the device was awarded the coveted CES® 2023 Best of Innovation Award in the digital health category, an award recognizing the best of breakthrough technologies improving health equity and saving lives.
This FDA-clearance is the first for the product and paves the way for expanded indications that encompass the full range of features offered by the platform. This includes the AeviceMD Monitoring System’s proprietary lung-sound analyzing algorithm which the company plans to clear in near future