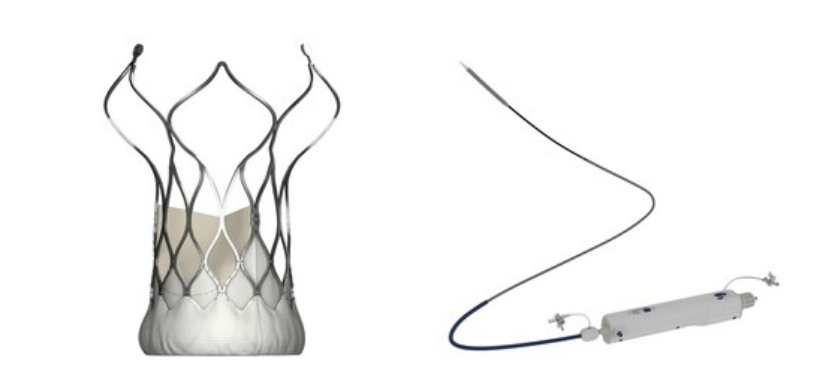
MicroPort CardioFlow Medtech Corporation, based in China, recently announced that its self-developed second-generation transcatheter aortic valve implantation (TAVI) device, the VitaFlow LibertyTM Transcatheter Aortic Valve and Retrievable Delivery System (VitaFlow LibertyTM), has received EU CE-MDR certification. This certification highlights VitaFlow LibertyTM as a pioneering TAVI solution, that sets a new benchmark in transcatheter heart valve treatments.
With over 47 million patients globally suffering from aortic valve stenosis and regurgitation, the prevalence rates of these conditions are on the rise due to an ageing population.
The TAVI solution provided by CardioFlow, which avoids open-heart surgery and offers various benefits like minimal trauma, quick recovery, and enhanced quality of life, is increasingly becoming a preferred choice for patients with aortic heart valve disease.
Before launching into the EU market, VitaFlow LibertyTM conducted pre-market clinical implantations at Galway University Hospital in Ireland, Rigshospitalet (Copenhagen University Hospital) in Denmark, and St Thomas' Hospital as well as Brighton & Sussex University Hospitals NHS Trust in the United Kingdom, and received very high appraisal from many well-known clinical professionals.