Findings from two groundbreaking studies highlight potential pharmacological and biosensor solutions for muscle mass preservation in patients undergoing obesity treatment therapy. Results from the BELIEVE study of bimagrumab and semaglutide combination therapy and a study of a novel continuous protein sensor for sarcopenia management were featured as a late-breaking symposium and late-breaking poster, respectively, at the 85th Scientific Sessions of the American Diabetes Association® (ADA) in Chicago.
“As we enter a new era of obesity treatments, it’s vital to focus not just on the amount of weight lost, but on preserving muscle mass and gaining the health benefits that result from treating obesity,” said Samar Hafida, the new Vice President of Obesity Association a division of the American Diabetes Association. “We are championing research to ensure people living with obesity can have access effective treatments to reduce adiposity while maintaining muscle mass critical to their well-being, and supporting durable long-term outcomes.”
The number of Americans on incretin-based therapies, such as glucagon-like peptide 1 receptor agonists (GLP-1 RA) and dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1RA has increased by 587% in the last 5 years. However, studies have shown that a reduction in muscle mass accompanies total weight loss. In fact, lean body mass can account for up to 15-40% of total weight loss from GLP-1 therapies.
Combination Therapy of Bimagrumab and Semaglutide Enhances Fat Loss and Preserves Muscle
Findings of study demonstrating the effectiveness of combining bimagrumab – a drug designed to combat muscle loss – with a common GLP-1 receptor agonist (RA), semaglutide, were presented during a late-breaking symposium.
The BELIEVE Phase 2b trial was a randomized, double-blind, placebo-controlled, multicenter study evaluating the effects of bimagrumab, alone and in combination with semaglutide, in adults with overweight or obesity. Bimagrumab is a first-in-class monoclonal antibody that targets activin type II receptors, promoting muscle preservation and growth. 507 participants received semaglutide as a once-weekly subcutaneous injection and/or bimagrumab administered via intravenous (IV) infusion at weeks 4, 16, 28, and 40. The primary endpoint was change in body weight (BW) from baseline. Secondary endpoints included changes in waist circumference, total body fat mass, visceral adipose tissue, and lean mass.
The results demonstrated the combination of bimagrumab and semaglutide therapy led to greater reductions in weight, body fat, visceral fat, and markers of inflammation compared to either treatment alone. The combination therapy yielded 92.8% of total weight loss from fat mass compared to semaglutide alone (71.8%) and a 22.1% decrease in bodyweight (−10.8% bimagrumab alone; −15.7% semaglutide alone). Notably, with the use of bimagrumab alone, 100% of weight loss was attributed to fat mass and there was an increase of 2.5% total lean mass.
"This study represents another major step forward in the evolution of obesity treatment, building on the significant weight loss benefits of semaglutide and combining it with bimagrumab to improve patient outcomes,” said Steven Heymsfield, MD, Professor at Pennington Biomedical Research Center and lead author of the study. “These insights indicate that is not only possible to achieve substantial fat loss, but also to preserve, or even enhance, lean mass in the process.”
The researchers are conducting studies of bimagrumab in combination with tirzepatide to evaluate its impact on both efficacy and safety.
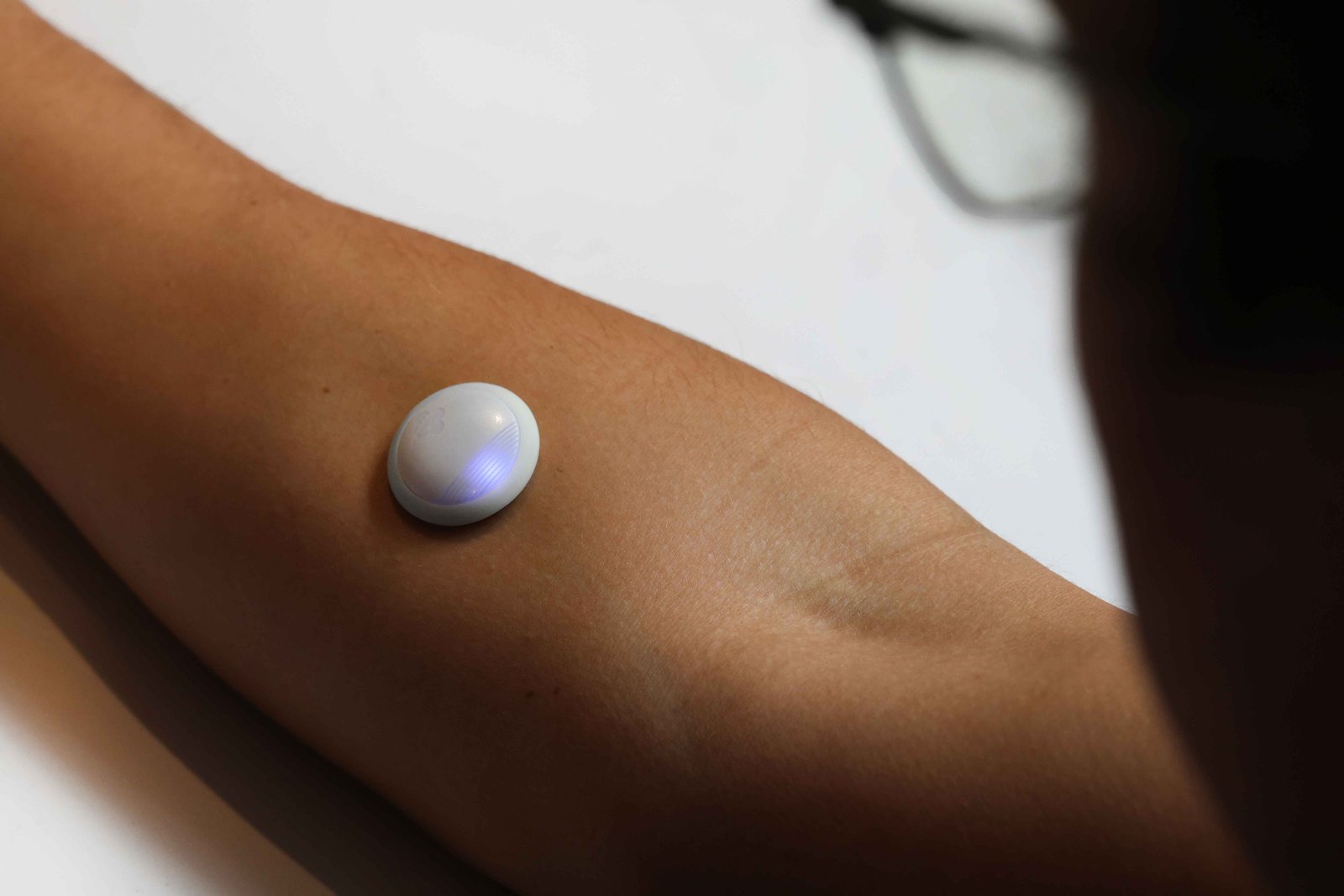
Novel Protein Sensor Shows Potential to Track Muscle Loss During GLP-1 RA Therapy
A new study, presented as a late-breaking poster, demonstrates proof of concept for a continuous protein sensor as a tool to prevent muscle loss during GLP-1 therapy.
The study evaluated a biosensor to track loss of lean muscle mass (LLMM) and protein ingestion in patients taking incretin based therapies. Using a DNA-based bioreceptor (called an aptamer), the sensor was designed to detect phenylalanine, and a key biomarker released during muscle breakdown or after eating protein.
While phenylalanine levels for healthy adults are 100 µM/L or higher with high protein meals, the sensor was tested in a lab with phenylalanine concentrations up to 1500 µM/L. Results showed the biosensor effectively detected phenylalanine with a low limit of detection (4 µM/L), within and beyond physiologic ranges. The findings indicate stability of the sensor over time, linear performance, and minimal sensitivity loss over seven days.
“GLP-1 medications have transformed the treatment of diabetes and obesity, but they can also increase the risk of muscle loss,” said Rebecca Gottlieb, Ph.D, Vice President of Advanced Sensor Technologies at Biolinq and lead author of the study. “While patients are advised to consume more protein to preserve muscle, it’s often difficult to know if they’re getting enough. That is why solutions to bridge this gap by providing real-time feedback are more important than ever — especially for those on GLP-1 therapies, older adults, or anyone managing sarcopenia.”
The next phase of research will focus on conducting clinical trials to evaluate its performance in real-world settings.
Research presentation details:
Dr. Heymsfield will present the findings at the late-breaking symposia presentation session:
Dr. Gottlieb will present the findings as a late-breaking poster session: