SeaStar Medical Holding Corporation, a commercial-stage therapeutic medical device company developing proprietary solutions to reduce the consequences of hyperinflammation on vital organs, has shipped QUELIMMUNE™ to a California medical center recognized as a world leader in pediatric research and treatments, increasing commercial customers to five. QUELIMMUNE is the company’s Selective Cytopheretic Device (SCD) for treating critically ill children in the intensive care unit (ICU) with acute kidney injury (AKI) and sepsis.
“We are delighted that QUELIMMUNE is now available to treat severely ill children at yet another prominent academic medical center,” said Tim Varacek, SeaStar Medical Senior Vice President, Commercial & Business Operations. “We are actively working to secure additional hospital clearances by gaining institutional review board (IRB) approvals for using QUELIMMUNE within their facilities. Currently, we are engaged with more than a dozen new medical centers, with seven of these hospitals advancing into the IRB approval process. Additionally, I’m pleased to report that several of our current customers have reordered QUELIMMUNE for their institutions since the beginning of this year.”
“We know of no other commercially available therapy that can modify the inflammatory process once it’s triggered to help repair the damage caused by an overactive immune system. It’s gratifying to expand access to our potentially lifesaving therapy to more pediatric patients as we make headway in our goal of having more than 20 hospitals utilizing QUELIMMUNE this year,” said Eric Schlorff, SeaStar Medical CEO.
QUELIMMUNE is being commercialized following U.S. Food and Drug Administration (FDA) approval for children with AKI and sepsis or septic condition weighing 10 kilograms or more who are being treated in the ICU with renal replacement therapy (RRT). QUELIMMUNE was approved under a Humanitarian Device Exemption (HDE) application, having met the applicable criteria with clinical results showing safety and probable clinical benefit in a limited population of critically ill children with AKI who have few treatment options.
Acute Kidney Injury (AKI) and Hyperinflammation
AKI is characterized by a sudden and temporary loss of kidney function and can be caused by a variety of conditions such as COVID-19, sepsis, severe trauma and surgery. AKI can cause hyperinflammation, which is the overproduction or overactivity of inflammatory effector cells and other molecules that can be toxic. Damage resulting from hyperinflammation in AKI can progress to other organs, such as the heart or liver, and potentially to multi-organ dysfunction or even failure that could result in worse outcomes, including increased risk of death. Even after resolution, these patients may face complications including chronic kidney disease or end-stage renal disease requiring dialysis. Hyperinflammation may also contribute to added healthcare costs, such as prolonged ICU stays and increased reliance on dialysis and mechanical ventilation.
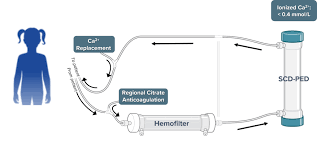
Selective Cytopheretic Device
The Selective Cytopheretic Device (SCD) is a patented cell-directed extracorporeal device that employs immunomodulating technology to selectively target proinflammatory neutrophils and monocytes during continuous renal replacement therapy (CRRT) and reduces the hyperinflammatory milieu including the cytokine storm. Unlike pathogen removal and other blood-purification tools, the SCD is integrated with CRRT hemofiltration systems to selectively target and transition proinflammatory monocytes to a reparative state and promote activated neutrophils to be less inflammatory. This unique immunomodulation approach may promote long-term organ recovery and eliminate the need for future renal replacement therapy (RRT), including dialysis.
The SCD has been awarded FDA Breakthrough Device Designation in four indications: