US-based Endogenex, a clinical-stage medical device company dedicated to improving outcomes in individuals with type 2 diabetes, has closed an oversubscribed Series C financing totaling $88 million.
The new capital will be used to complete the pivotal ReCET Clinical Study, which has been granted an Investigational Device Exemption (IDE) by the US Food and Drug Administration (FDA).
A group of new investors, including Hatteras Venture Partners, Lumira Ventures, and Orlando Health Ventures, joined an undisclosed strategic lead investor and existing investors Intuitive Ventures, Longitude Capital, Mayo Clinic, and Santé Ventures in the Series C funding.
The ReCET Clinical Study is a multicenter, prospective, randomized, double-blinded, sham-controlled study assessing the safety and effectiveness of the ReCET System. The pivotal study received IDE approval in November 2023. The study will enroll up to 350 patients at clinical sites in the United States and Australia.
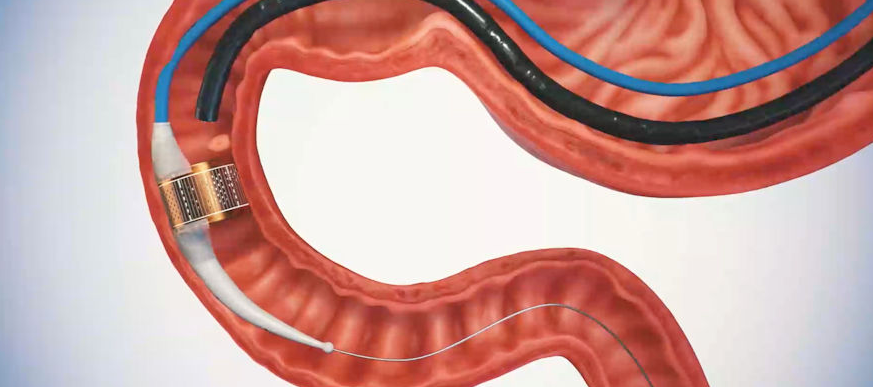
ReCET is a novel, endoscopic outpatient procedure that targets the cellular pathology of the duodenum. This pathology may contribute to the development and progression of type 2 diabetes.
The ReCET System has received an US FDA Breakthrough Device Designation for treating type 2 diabetes in adults inadequately controlled by glucose-lowering medications.