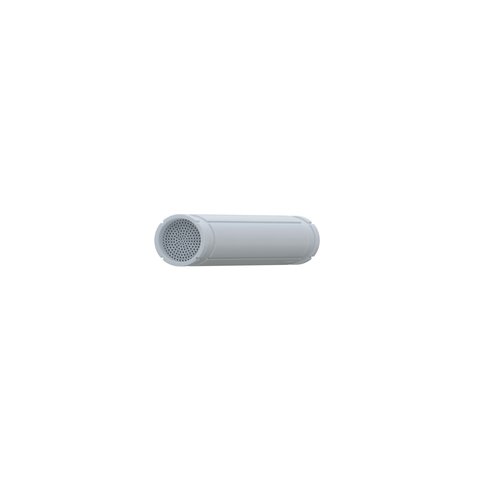
Auxilium Biotechnologies has launched a groundbreaking clinical trial to evaluate its innovative nerve regeneration device, NeuroSpan Bridge™, marking a pivotal moment in regenerative medicine. This investigational device is designed to accelerate peripheral nerve repair by guiding and supporting nerve regeneration, potentially restoring motor and sensory functions more effectively than current surgical techniques. By using a bioengineered conduit that mimics natural nerve pathways, NeuroSpan Bridge aims to reduce complications such as chronic pain and improve overall recovery outcomes for patients with traumatic nerve injuries.
The Neurospan-1 trial, a multicenter study aiming to enroll 80 patients across the U.S., will assess the safety, efficacy, and long-term benefits of the device. NeuroSpan Bridge is especially suited for patients with complex nerve damage in the extremities resulting from car accidents, workplace trauma, or surgical injury. The device has been designed for ease of use in clinical settings and offers an advanced alternative to autografts or synthetic conduits, which often fall short in delivering consistent regenerative results. If successful, the trial could significantly influence how clinicians approach nerve repair.
This clinical advancement not only signals Auxilium's commitment to innovative, life-changing therapies, but also reinforces its leadership in regenerative technology. The company’s broader expertise, including its work in microgravity bioprinting on the International Space Station, reflects a unique and visionary approach to medical device development. The successful execution of the Neurospan-1 trial may pave the way for FDA approval and widespread adoption, offering new hope to thousands of patients affected by nerve injuries and setting a new standard in neuromuscular care.
MedTech Spectrum's Summary
NeuroSpan Bridge™ offers a breakthrough in nerve regeneration by accelerating repair and reducing complications like chronic pain, improving recovery for patients with traumatic nerve injuries.
The Neurospan-1 clinical trial will evaluate the device’s safety and efficacy in real-world settings, potentially transforming standard treatment for peripheral nerve damage.
Auxilium’s innovation reflects its commitment to advancing regenerative medicine, with the trial’s success paving the way for broader adoption and improved patient outcomes worldwide.