Cervos Medical has reached a significant milestone with the EU MDR certification of its CERVOS® KeyPRP System, affirming the device's adherence to the European Union’s highest standards for medical device safety, performance, and quality. The certification under EU MDR 2017/745 is a rigorous validation that reinforces the system's credibility and regulatory strength, distinguishing it within the competitive regenerative medicine landscape. This achievement reflects Cervos Medical’s commitment to excellence in manufacturing and quality assurance, and sets the foundation for broader market penetration across Europe.
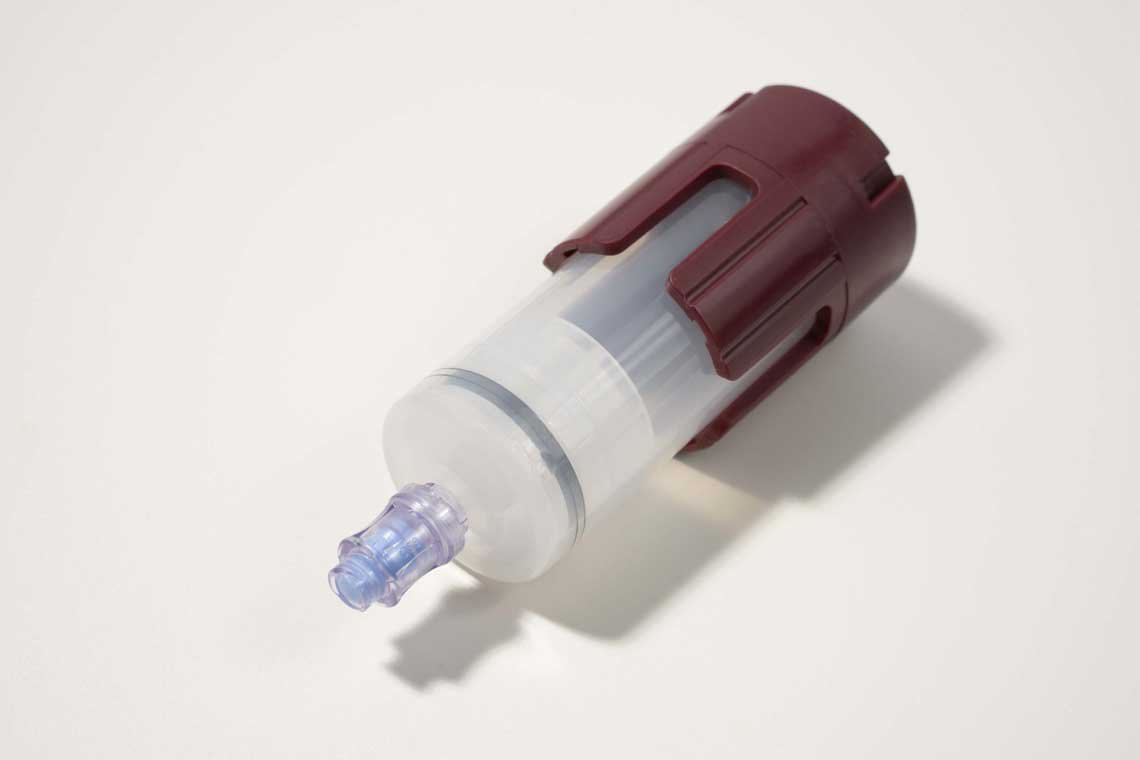
The CERVOS® KeyPRP System is a state-of-the-art platelet separator designed for use in autologous regenerative procedures, including platelet-rich plasma (PRP) therapy, bone marrow aspiration, and adipose tissue processing. It supports clinicians in delivering personalized, cell-based treatments with enhanced efficiency and precision. By enabling clinicians to concentrate and prepare PRP from the patient’s own blood, the system enhances healing and recovery outcomes in musculoskeletal, orthopedic, and aesthetic procedures. Its design emphasizes safety, sterility, and usability, aligning with the evolving clinical needs for minimally invasive, regenerative solutions.
With CE certification in place, Cervos Medical is poised for expanded adoption of the KeyPRP System in the European market, distributed exclusively by Aspire Medical. This strategic milestone underscores the company’s readiness to meet growing global demand for advanced regenerative tools. Backed by parent company Ranfac Corp, Cervos continues to lead innovation in autologous tissue processing, offering solutions that enhance both clinical performance and patient care. The EU MDR approval not only legitimizes its quality but also accelerates the system’s path toward broader clinical impact and international reach.