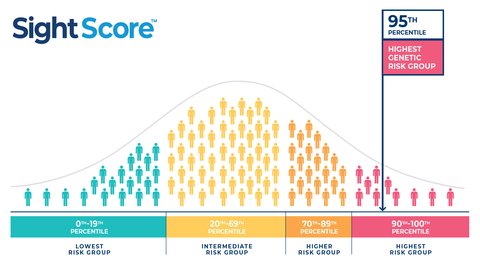
Seonix Bio has announced the U.S. launch of SightScore™, the first commercially available clinical polygenic risk score (PRS) test for glaucoma. This groundbreaking test enables early risk assessment, helping eye care professionals identify individuals at a higher genetic risk for primary open-angle glaucoma (POAG) before symptoms develop.
SightScore™ leverages advanced genetic insights to offer a personalized approach to glaucoma management, supporting proactive screening and tailored monitoring strategies. By integrating PRS testing into clinical practice, Seonix Bio aims to transform glaucoma care, improving early detection and reducing the risk of irreversible vision loss.
With glaucoma being a leading cause of blindness worldwide, the launch of SightScore™ represents a major step forward in precision eye care. The test is now available to healthcare providers, empowering them to make data-driven decisions and enhance patient outcomes.