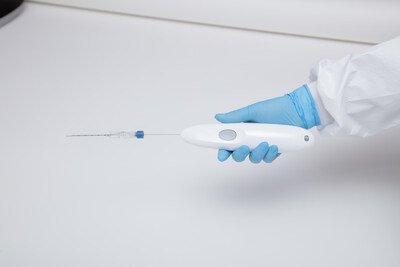
Single Pass, with the first and only biopsy closure cauterization device, is delighted to announce the successful completion of the first real- life cases utilizing the groundbreaking device. These cutting-edge procedures, conducted at the Universitario Campus Bio Medico, Fondazione Policlinico in Rome, Italy, demonstrate the device's exceptional capabilities and potential to revolutionize the field of biopsy closure.
In the initial case, a kidney biopsy was performed under CT guidance utilizing the 22cm Single Pass KRONOS device and a 13cm biopsy needle. The procedure resulted in successful cauterization with a CT-guided determination of the target lesion. The insertion of the Single Pass KRONOS probe seamlessly connected the biopsy needle with the depth gauge, ensuring precise and accurate results.
In the second case, an 80-year-old female underwent a liver biopsy utilizing a 17cm Single Pass KRONOS device and ultrasound guidance. Despite substantial post-tissue sample removal bleeding, the device provided excellent visibility, enabling the medical team to achieve perfect track closure and full cauterization. The Single Pass device design allowed for exceptional visual feedback and ease of tracking under ultrasound guidance. Each primary physician operator praised the usability and performance of the Single Pass KRONOS device. In both instances, the device successfully cauterized the biopsy channel, effectively stopping any post-biopsy bleeding.
The success of these initial real-life cases highlights the immense potential of the Single Pass KRONOS biopsy closure cauterization device. With its exceptional performance and ease of use, the Single Pass KRONOS device is poised to revolutionize the field of biopsy closure.