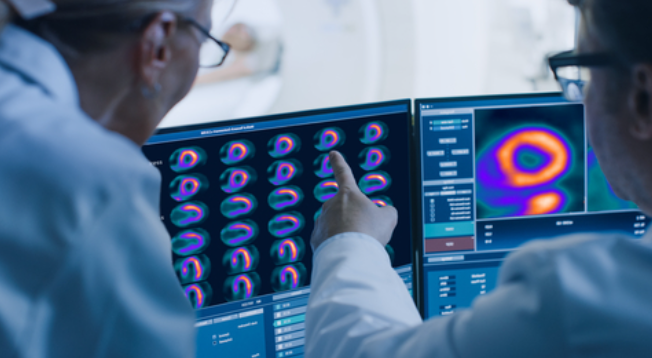
GE HealthCare (Nasdaq: GEHC) today announced that the U.S. Food and Drug Administration (FDA) has granted approval of Flyrcado (flurpiridaz F 18) injection, a first of its kind positron emission tomography myocardial perfusion imaging (PET MPI) agent, for the detection of coronary artery disease (CAD). Indicated for patients with known or suspected CAD, Flyrcado delivers higher diagnostic efficacy compared to single-photon emission computed tomography (SPECT) MPI, the predominant procedure used in nuclear cardiology today. Flyrcado, which can be manufactured in an offsite pharmacy and delivered as a ready-to-use unit dose, has the potential to expand clinician and patient access to PET MPI, including improving diagnostic accuracy in difficult-to-image patients such as those with a high body mass index (BMI) and women1.
With a half-life of 109-minutes—significantly longer than existing PET MPI tracers—Flyrcado removes the need for on-site tracer production and generator maintenance and enables distribution to a wide network of hospitals and imaging centers. This longer half-life also means Flyrcado brings the first practical opportunity to combine exercise stress testing with cardiac PET imaging for CAD, enabling the most robust protocol for evaluating ischemia in patients. Furthermore, clinicians would have the ability to rescan a patient during the same imaging session in the event of technical difficulties, rather than rescheduling an additional scan.
Flyrcado is one of three F 18 imaging agents in GE HealthCare’s portfolio of FDA-approved molecular imaging PET products, joining radiopharmaceutical PET imaging agents Cerianna (fluoroestradiol F 18) injection, used to detect estrogen receptor positive lesions as an adjunct to biopsy in patients with metastatic and recurrent breast cancer, and Vizamyl (flutemetamol F 18) injection which is a PET tracer for imaging of the brain to estimate beta amyloid neuritic plaque density in adult patients who are being evaluated for Alzheimer’s disease or other causes of cognitive decline.
GE HealthCare acquired exclusive global commercialization rights for flurpiridaz F 18 from Lantheus in 2017 and has led the funding and development of the product through to approval. Lantheus collaborated on the development and will also collaborate on commercialization through a joint steering committee.
Flyrcado will be available in initial U.S. markets in early 2025 with expanding availability thereafter.