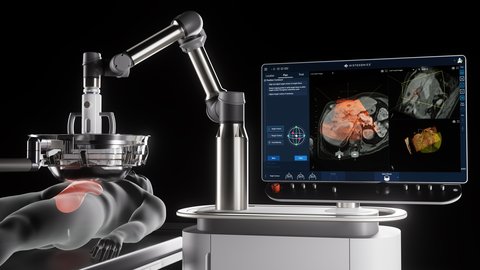
HistoSonics’ Edison Histotripsy System introduces a groundbreaking non-invasive approach to tumor treatment using focused ultrasound technology known as histotripsy. Unlike traditional cancer treatments such as surgery, radiation, or chemotherapy, histotripsy uses mechanical forces created by focused sound waves to liquefy tumors at the cellular level. This technology enables physicians to precisely target tumors without damaging surrounding healthy tissues and offers continuous real-time imaging and control during the procedure. Its De Novo clearance by the U.S. FDA in 2023 and now its early access authorization in Great Britain underscore its global potential as a disruptive force in cancer treatment.
The Edison System is specifically designed for the destruction of primary and metastatic liver tumors in patients who may not be candidates for surgical resection or other invasive treatments. Through the UK’s Unmet Clinical Needs Authorisation (UCNA) pathway, patients in Great Britain now have early access to this novel therapy. Key benefits include a non-thermal, scar-free procedure that reduces recovery time and eliminates the common side effects of radiation or systemic drugs. Its real-time monitoring ensures accurate targeting and complete treatment, helping reduce recurrence risk and improve clinical confidence.
This early UK market entry not only highlights the Edison System’s potential to address urgent oncology care gaps but also sets a precedent for accelerated adoption of novel devices that meet high-priority health needs. Its selection for the UK's Innovative Devices Access Pathway program places HistoSonics among the most promising technologies poised to transform patient outcomes. With broader clinical trials underway for kidney and pancreatic tumors, and efforts to secure CE marking for European expansion, the Edison Histotripsy System is well-positioned to redefine standards of care for hard-to-treat cancers around the world.