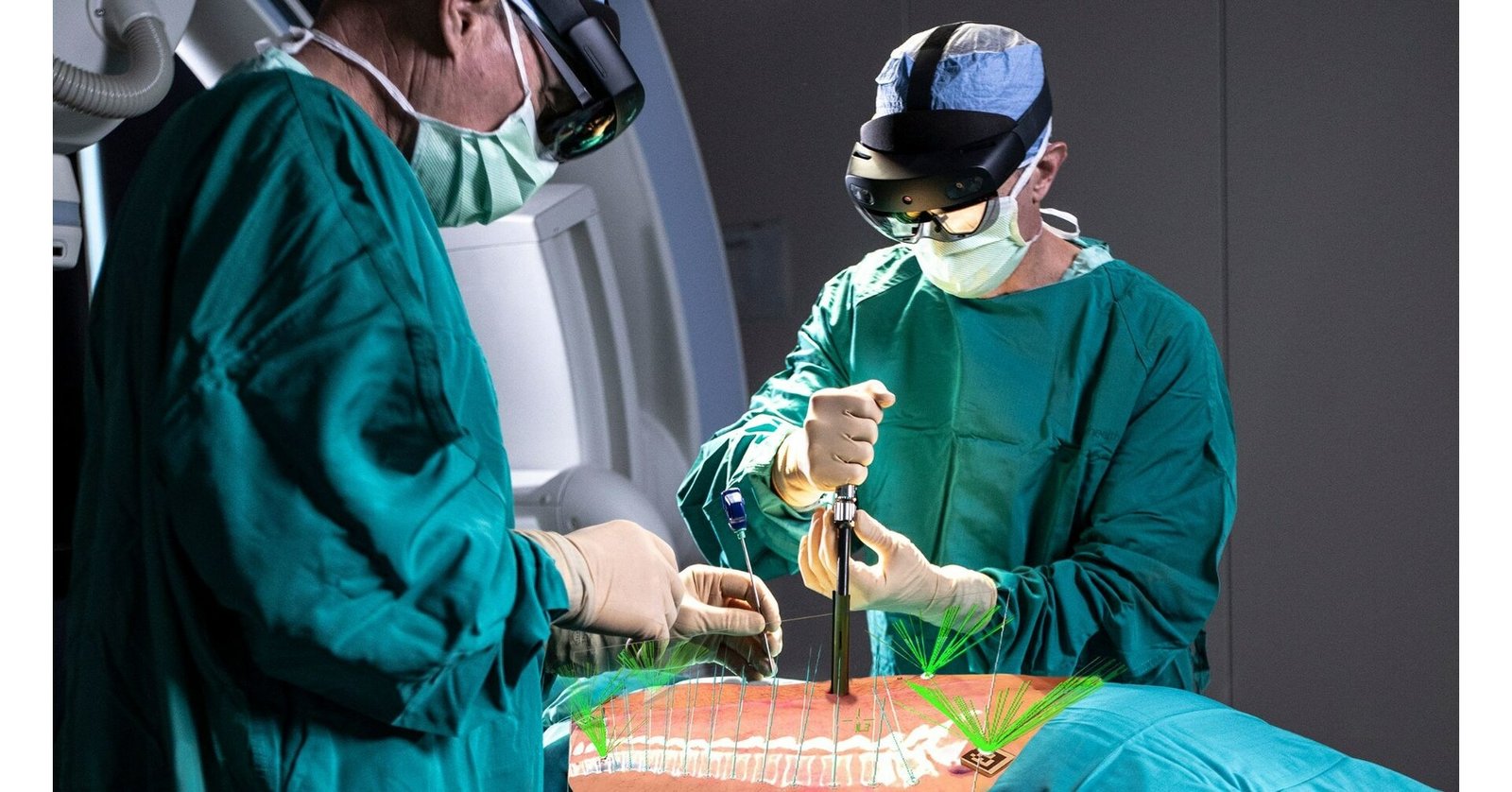
US-headquartered medtech firm Novarads VisAR, a surgical navigation system that uses augmented reality (AR), has received clearance from Indonesias Food and Drug Administration (FDA) for intraoperative use in stereotactic spinal surgery.
VisAR is accurate for both open and minimally invasive surgery (MISS). This cutting-edge technology enables surgeons to transform a patients imaging data into a 3D hologram, which can be projected onto the patients body with extreme precision. This allows surgeons to concentrate solely on the surgical objective without requiring them to look away towards a separate monitor, providing accurate surgical guidance.
The VisAR system is a comprehensive solution that includes pre-surgical planning, virtual annotations, segmentation, as well as two-way image connectivity. It also provides integrated 2D and 3D immersive navigation views and ongoing hologram-to-patient registration. VisAR has a sub-2 mm accuracy rate for pedicle screw placement in open and minimally invasive surgical procedures. With a fast a seamless set-up, VisAR enables the surgeon to view the entire OR footprint with voice-controlled commands.
Novarad has collaborated with Microsoft to utilise pre-built AR headset technology, allowing for lower cost and the ability to leverage expected hardware advancements. With VisAR, physicians wear the wireless Microsoft HoloLens 2 visor and have no need for any other navigational equipment.